FUTURISTIC FIXATION AVAILABLE TODAY
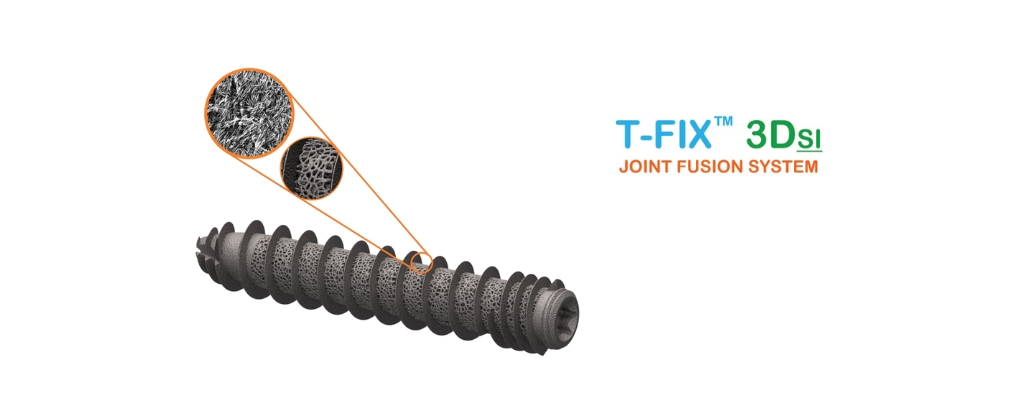
The 3D printed T-FIX™ 3DSI Joint Fusion System, intended to treat dysfunction of the sacroiliac joint, is a patented ‘passively smart’ SI Joint Fusion System designed with an emphasis on optimal osseointegration as supported by solid science.
With 510(k) clearances for 4 critical approaches to the sacroiliac joint, the T-FIX™ 3DSI Joint Fusion System is not only transformational in design but also transformational relative to being able to offer four approaches with one system; ultimately providing optimal fiscal value to those that partner with Cutting Edge Spine.
"HAVING A ‘PATIENT FIRST’ MENTALITY AND ABIDING BY EXTREME ETHICS RELATIVE TO SURGEON RELATIONSHIPS IS AT THE CORE OF OUR ORGANIC DEVELOPMENT INITIATIVES."
Randy Roof – President & CEO; Founder
REQUEST MORE INFORMATION
WHY CUTTING EDGE SPINE
CES provides the spine marketplace with transformational spinal technologies that are ‘passively smart’ in nature in addition to offering extensive experience as a Team relative to organic product development, regulatory affairs, materials science, biomechanical engineering, product commercialization and supply chain management.
OUR 510(k) PORTFOLIO INCLUDES
- T-FIX™ 3DSI Joint Fusion System
- EVOL®SI Joint Fusion System
- EVOSha Lumbar Interbody System
- EVOL®ha-C Cervical Interbody System
- EVOL®ha-D Direct Lateral System
- nuMIS™ semi-rigid lumbar plating system




