POROUS ORTHOFIXATION HOLDINGS DIVISION
Home to Cutting Edge Spine’s intellectual property relative to trabecular fixation for all orthopedic applications, and under the guidance of Chairperson Lisa Ferrara, PhD, and its Surgeon Advisory Panel, the Porous Orthofixation Holdings division of the company is devoted to the development, testing, and licensing of new applications relative to fixation that leverage the company’s strong patent position.
If you have an interest in learning how we can apply your trabecular concepts to our patented fixation platform please contact us.
Issued Patent Numbers:
10,993,754 IMPLANTS FOR TISSUE FIXATION AND FUSION
11,446,070 IMPLANTS FOR TISSUE FIXATION AND FUSION
11,771,482 IMPLANTS FOR TISSUE FIXATION AND FUSION

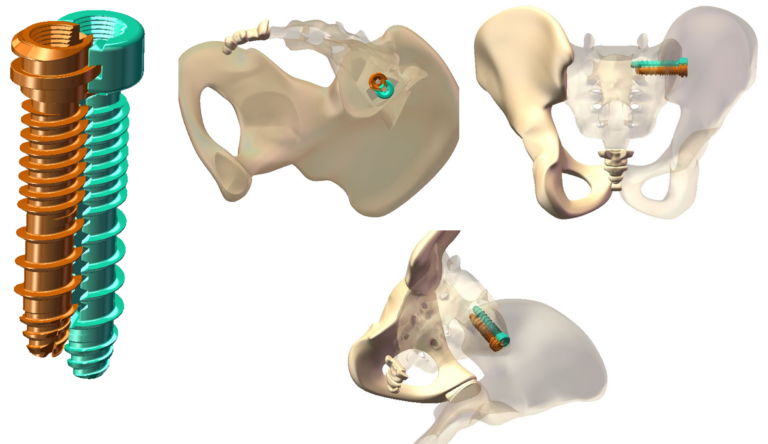
Dual Screw Technology
Minimal disruption with maximum fixation!
Issued Patent Numbers:
10,588,750 IMPLANTS AND TECHNIQUES FOR TISSUE FIXATION AND FUSION
11,654,028 IMPLANTS AND TECHNIQUES FOR TISSUE FIXATION AND FUSION
Dedicated to meeting the needs and expectations of its customers while developing marketing, and selling innovative products and services.
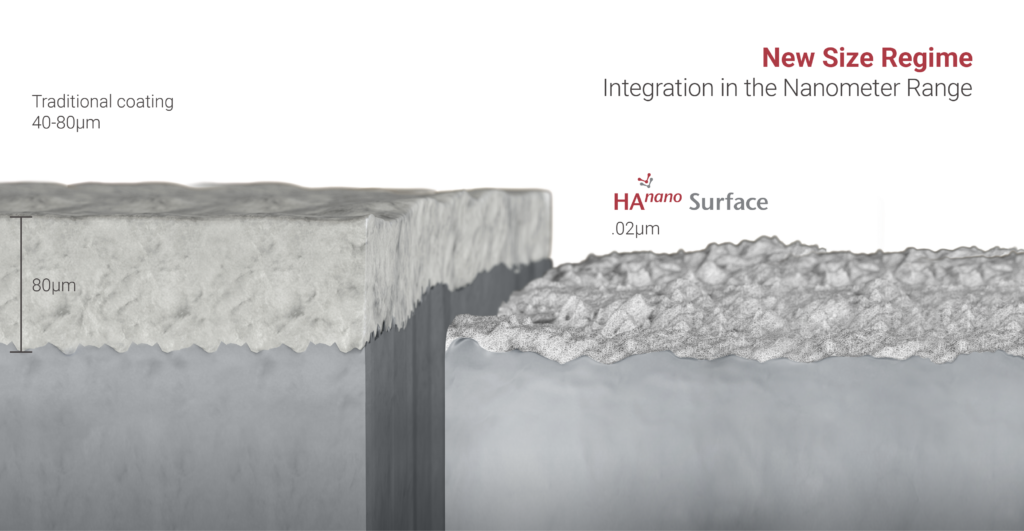
HAnano SURFACE ®:
As the first entity in the world to receive a non-dental (Brazil) regulatory approval for an orthopedic device applying this novel HAnano Surface, Cutting Edge Spine has a level of experience relative to this particular technology that is extensive in nature having collected clinical data since 2019 and extensively studying the application of the technology to various orthopedic implant forms.
INVIBIO HAenhanced ®:
As the first entity in the U.S. to receive a 510(k) clearance with HAenhanced for lumbar spinal application(s), with clinical data on our EVOSha lumbar interbody system presented at NASS in 2016 & 2017, Cutting Edge Spine is not only proud to be a pioneer in said regard but also steadfastly committed to the material given its many positive characteristics relative to interbody application(s). At present Cutting Edge Spine, arguably, offers the absolute largest portfolio of HAenhanced® interbodies in the world.

